Experiment: Try sticking 2 strips of scotch tape together and quickly peeling them apart. They should be attracted to each other. Repeat the experiment again to make 2 more strips. Some of the new tape will now repel the old tape.
These experiments can be explained if we imagine a substance called electric charge. When you rub some materials together the charge separates into 2 types. Each type of charge is attracted to the opposite type and repelled by the same type. It turns out those simple rules also explain electricity, chemical bonds, magnetism, and light!
opposite charges have an attractive force
negative charges have a repulsive force
positive charges have a repulsive force
neutral or balanced charges have no net force
If a positive and negative charge are close together the attractive and repulsive forces mostly cancel each other out. This explains why we don't notice the electrostatic forces until charges get separated.
Electric charge can't be created or destroyed. Like energy and momentum, electric charge is conserved.
The original evidence for this conservation law was based on repeated experiments. No one ever documented the total charge of a system increasing or decreasing. Charged particles can be created and destroyed, but only when another particle is created or destroyed to balance out the total charge.
symmetry and conservation laws
Conservation of charge, energy, and momentum are laws. Laws are just patterns we see when we collect data. We didn't have a explanation for these conservation laws until 1915 when Emmy Noether published a mathematical proof that conservation can be understood as a consequence of symmetry.
Symmetry is a property that doesn't change after a transformation. Charge is conserved because of a symmetry of electromagnetic fields called gauge invariance. This is a quantum mechanical principle related to magnitude and phase of a wave function.
There are a few different conservation laws in classical physics:
You might have heard of conservation of matter, but matter isn't always conserved. You can destroy or produce matter by converting it into energy.
Elementary Electric Charge
Charge is measured in Coulombs (C). Charge increases the strength of the electrostatic force in the same way that more mass increases the strength of the gravitational force. We'll learn more when we study Coulomb's law.
In 1909 Robert Millikan and Harvey Fletcher performed the oil drop experiment to investigate electric charge. They sprayed a fine mist of oil into a uniform electric field. The electric field produced a force on some of the oil droplets. Based on that force, they found that charge only came in even multiples of about 1.6 × 10−19 C.
This evidence shaped the early model of the atom: negative electrons bound to a tiny nucleus of positive protons and neutral neutrons. Pretty much all charge that exists comes from electrons and protons, but there are some rare exotic charged particles.
charge = −1.602 × 10−19 C
mass = 9.109 × 10−31 kg
charge = +1.602 × 10−19 C
mass = 1.672 × 10−27 kg
strategy
Charge only comes in even multiples of ±1.6 × 10−19 C.
You can't have half a charge, but you could have 3 charges.
solution
Charge has only been observed in packets of 1.602 × 10−19 C. Any recorded charge must be a multiple of this value.
solution
Use a conversion fraction with 1 electron and the charge on an electron.
$$-4.5 \, \mathrm{C} \left(\frac{1 \, \mathrm{e^-}}{-1.6 \times 10^{−19} \, \mathrm{C}}\right) = 2.81 \times 10^{19} \, \mathrm{e^-}$$solution
$$234 \times 10^{12} \, \mathrm{e^-} \left(\frac{-1.6 \times 10^{−19} \, \mathrm{C}}{1 \, \mathrm{e^-}}\right) = -3.744 \times 10^{-5} \, \mathrm{C}$$Conductivity
Conductive materials allow electric charges to easily move through them. Stuff related to the motion of charge is called electricity.
If a charge is applied to one part of a conductive material the charge will quickly spread out because like charges repel.
In chemistry, elements are roughly divided into metals, metalloids and nonmetals. Metals are held together by loosely sharing their outer valence electrons. The cloud of free flowing electrons give metals most of their shared characteristics, like conductivity.
insulators
vacuum, nonmetals: gases, plastics, silk, fur
electrolytes
solvents with dissolved ions:
salt water, tap water, soda water
semiconductors
metalloids: carbon and silicon
conductors
metals, plasma
superconductors
certain low temperature ceramics
air, coca cola, copper, carbon, plastic fork
answer
high conductivity
copper (conductor)
carbon (semiconductor)
coca cola (electrolyte)
plastic fork (insulator)
air (insulator)
low conductivity
answer
In metals some electrons are free to move between atoms. In nonmetals the electrons are locked up in covalent bonds so they resist the electrostatic force.
Another way to make a substance conductive is to heat it up so much that electrons can leave the nucleus. We call this state of matter a plasma.
Static Electricity
It's easy to separate a couple trillion electrons from their protons by walking with socks on a carpet. Lightning is produced in a similar way when a cloud with rising air ends up with an unbalanced distribution of charge.
Static electricity occurs when there is an imbalance of electrons and protons. A lasting charge separation can only occur in insulating materials, because in conductors positive and negative charges quickly pair up.
Static electricity effects are much stronger and longer lasting in low humidity. This is because water molecules increase the conductivity of air, allowing more separated charges to return.
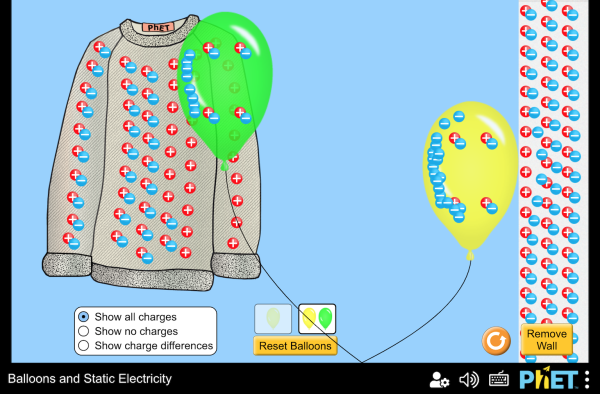
Play around with this PhET simulation for static electricity.
Question: Which are conductors and which are insulators?
(balloons, sweater, wall, air)
answer
conductors: nothing in this simulation
insulators: balloon, sweater, wall, air
Why not the other way around?
answer
Some materials are better at holding onto extra electrons for complex quantum mechanical reasons.
answer
After rubbing, the balloon has unpaired negative charge, and the sweater has unpaired positive charge. Opposite charges attract.
answer
After rubbing, the balloon has unpaired negative charge. When charge is near another insulator it repels the electrons enough that they are slightly farther away, but not enough to cause them to leave the nucleus. This charge separation is called polarization.
The polarization of the positive and negative pairs creates an induced charge. The charged balloon causes the wall to polarize, which increases the attraction and decreases the repulsion between the balloon and wall.
A TriboElectric Series lists which materials will become electrically charged after they are rubbed together.
Question: If you rubbed rubber on a cat, static electricity would cause them stick together. Which would gain a positive charge?answer
The cat would end up with a positive charge. This means the cat would lose electrons to the rubber.
answer
The glass would get the positive charge.